8. Tissue Engineering
Problem 1: Design considerations for electrical stimulation systems
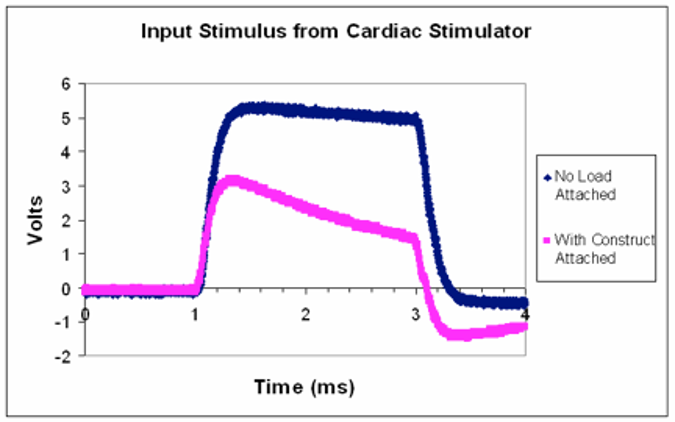
The stimulus waveform is approximately that of a square pulse of 5V amplitude. Describe what happens to the stimulus waveform with the bioreactor attached.
The amplitude drops when the bioreactor is attached.
Assuming there is a single impedance value for a single bioreactor can you think of an explanation for why the stimulus waveform might be distorted when connected to the load?
How might you expect the pink waveform to change if several of the same bioreactors were to be attached in parallel to this stimulator?
The amplitude would be further decrease, or disappear?
What recommendation for modifying the stimulator would you make to solve this problem?
Ohm’s law: V = IR
So we can raise the stimulator current (I)
Problem 2: Morphological changes associated with electrical stimulation
I downloaded and used ImageJ. I tried to apply various filters, edge detctors and more to nicely segment the cells. I couldn’t find myself any steps that helped out, so I found the paper Quantification of fluorescence intensity of labeled human mesenchymal stem cells and cell counting of unlabeled cells in phase-contrast imaging: an open-source-based algorithm. (Polzer 2010) which contained a detailed algorithm to follow a similar task:
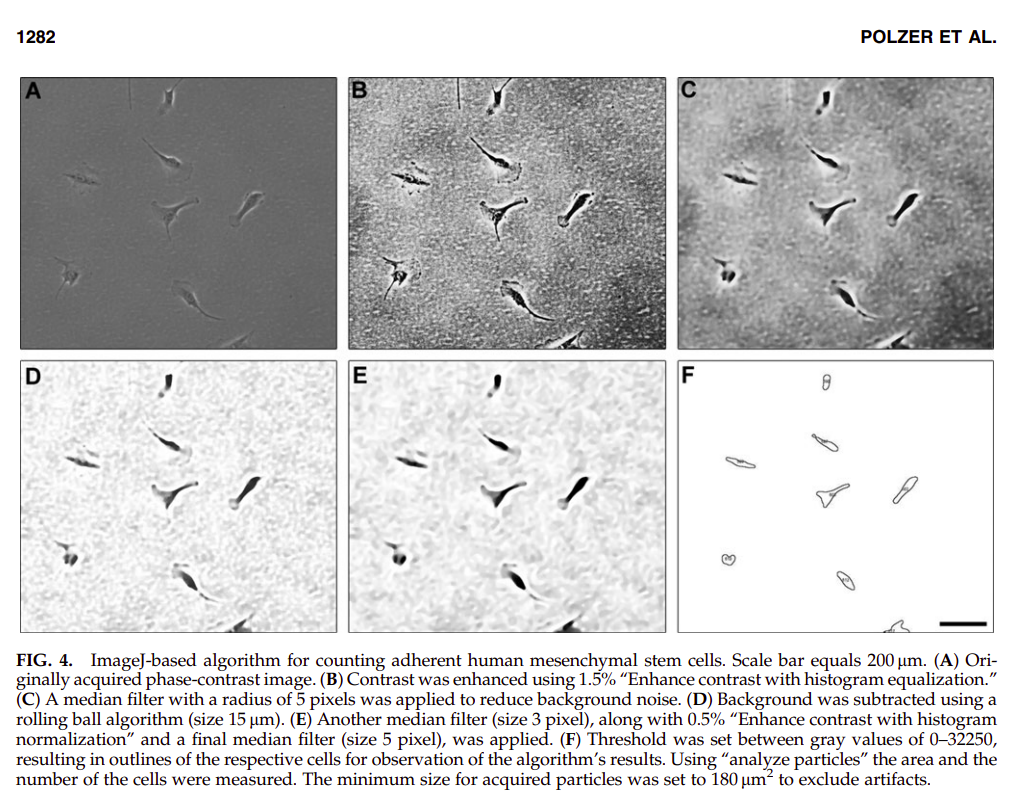
So I tried to apply the same steps to our images, starting with ES- t=0:
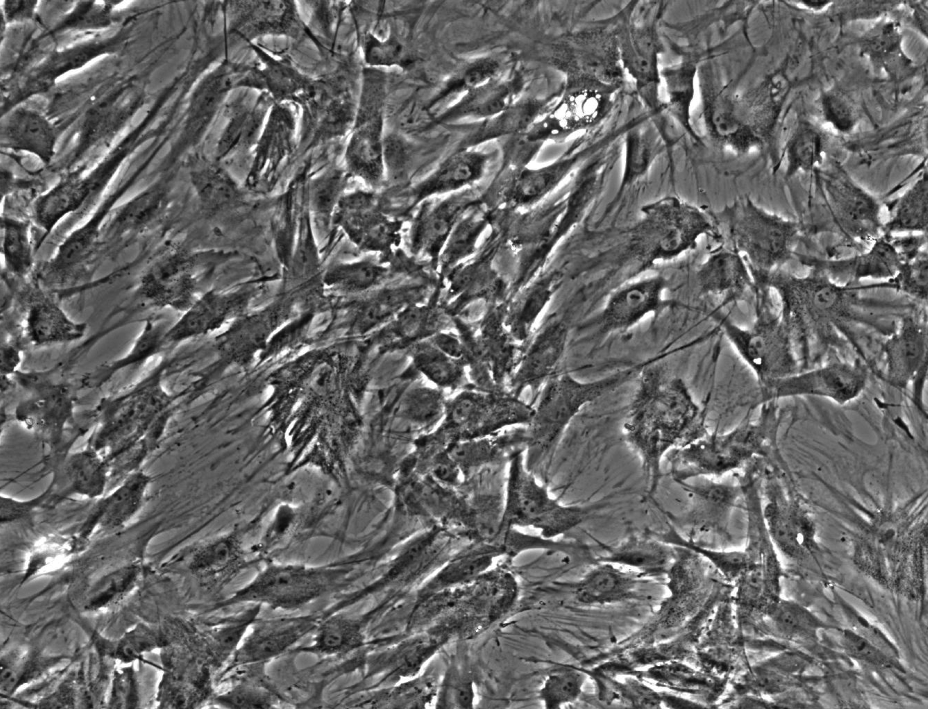
Enhanced Contrast (5%) with histogram equalization:

Median Filter (8px) & Rolling Ball Background Subtraction:
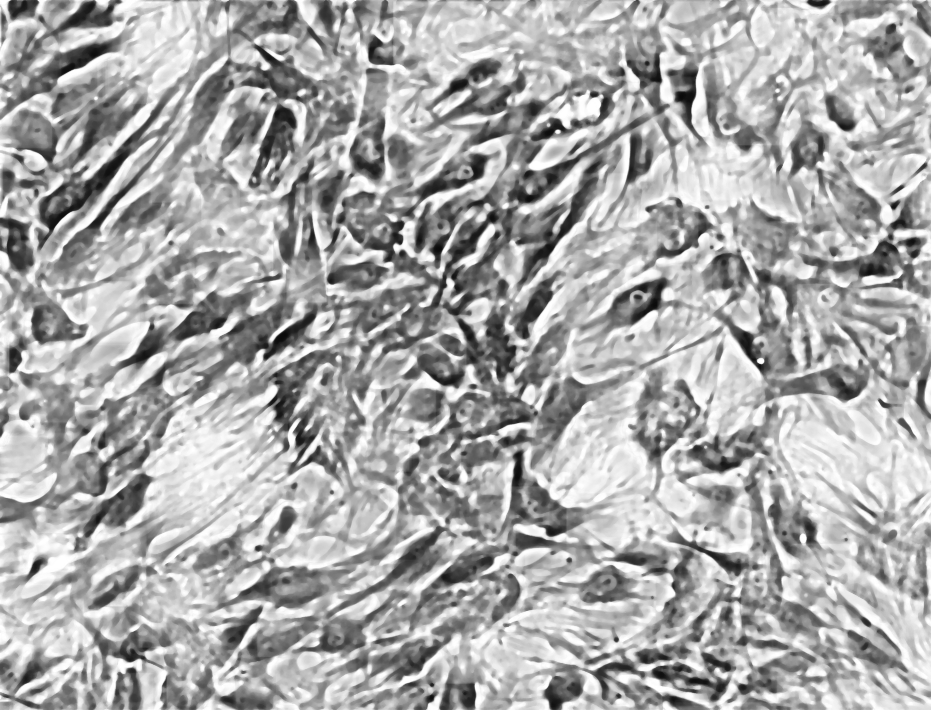
Median Filter 5px, Enhance Contrast 1%, Median Filter 8px:
Threshold:
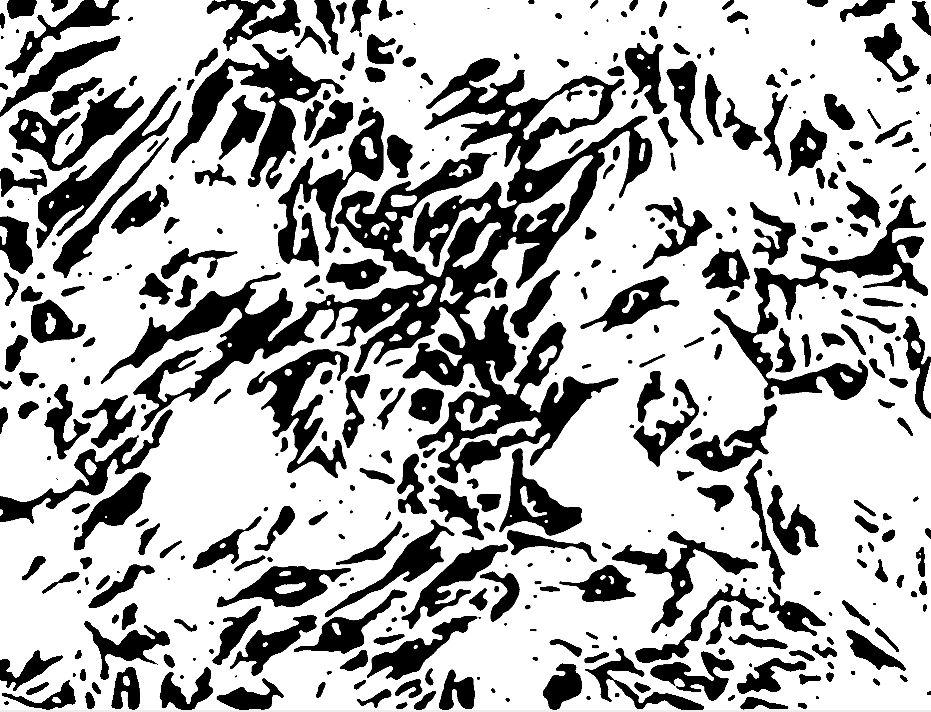
And analyze particles did not work… as you can see the result is not easily segmentable yet. I could manually go over the cells and measure each. If the cells were less densely packed it would have been easier to segment. Nonethless, I think it would be possible to write a computer vision algorithm to solve it…
Problem 3: design considerations for perfusion bioreactors
When we grow tissues outside their natural habitat (in vitro), it’s critical to try and mimic the physiological conditions the tissue is “used to” grow at. The worked example in pp 1191-1193 explains how to estimate the flow rate, of a given perfusion bioreactor, such that the tissue growing inside that reactor will grow in conditions most similar to a real human body, in this case, under low and high shear stress.
There are a few assumption in the calculation:
- The bioreactor has six channels.
- Each channel has a scaffold on it. The scaffold is modeled as a cylinder of 5mm height and diameter of 5mm. The channels inside the scaffold are modeled as cylinders of 5mm height and 400um diameter.
- Flow is assumed to be laminar (non turbulent) upwards.
The scaffold is assumed to e 70% porous. So the void volume is approximated the volume of the cylinder times 0.7 = 13.74 mm^2
The fluid velocity is approximated under low (1 dyne/cm^2) and high (10 dyne/dm^2) shear stress conditions as 0.649 cm/s and 6.49 cm/s respectively.
The geometry of the scaffold is assumed such each scaffold contains cylinder channels that makes it 70% porous. Thus every scaffold has 109 channels.
In reality, it’s hard to imagine that porous scaffolds would create such perfect straight channels. I would assume that over time that channels become less linear, as well as interconnected, thus we need to account for inter-channel fluid dynamics. The above approximation make up for a good upper bound. Perhaps another analysis that assumes a very porous interconnected model could be used to asses a lower bound on the same parameters.

According to the above figure, it seems that some areas of this tissue experience 10x more flow/shear stress compared to other areas in the tissue. Some areas barely have any flow even. We might expect then, for tissues with complicated geometries, over time different regions of the tissue will mature differently, and we cannot expect the same cell quality across the tissue. This conflicts with the simple model suggested before of nice cylinders and laminar flows.
DIY decellularized scaffolds!!
This is cool. We are going to (try) and make decellularized scaffold from old food in hours homes. While I was curious to try these old bacon lamps, I decided to stick with any organic food I had which happened to be:
- Eggplant
- Bell pepper
- Onions
- Garlic
- Spinach
- Sweet Potato
So I cut each of these, some in the approximate shape of an ear, as a nod to Andrew Pelling, and some in the shape of hearts, as a nod to this ghost heart video

Put in for 40 minutes at the freezer (yes, my freezer is that empty I didn’t have to take anything out :) :( )

Then I prepared tap water with dish soap (contains sodium lauryl sulfate, about 3 squeezes to a tall IKEA lunch box filled with water) and pured into specialized containers:


I started this on Sunday 04/19 night…
The next day, most veggies remained changeless exacpt the pioneer eggplant, which lost a lot of color in the body and the peel:

I switched water with soap 3 times during that day… with the eggplant still as the main promise.. but with the onion catching on.
On Tuesday:

